Clinical commitment
Termosalud has a clinical department, staffed by doctors and experts in the sector who evaluate and guarantee the efficacy and safety of our medical and medical-aesthetic devices on a daily basis.
Our clinical commitment ranges from rigorous compliance with the ISO 13485 standard referring to the quality management system applicable to medical devices, to the development of scientific studies monitored by the Ethical Committee of Asturias and in compliance with the Declaration of Helsinki.
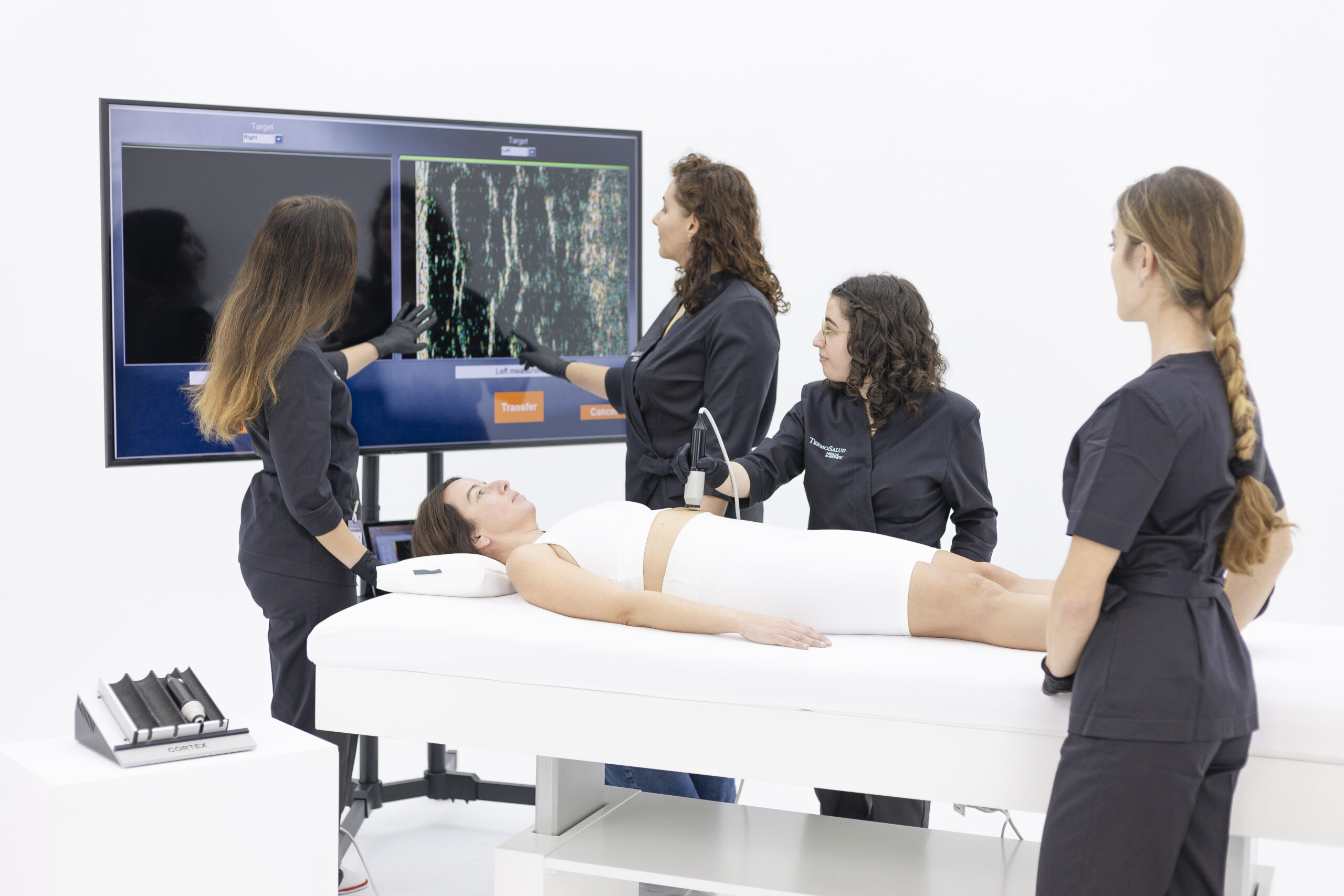
TERMOSALUD_Clinic
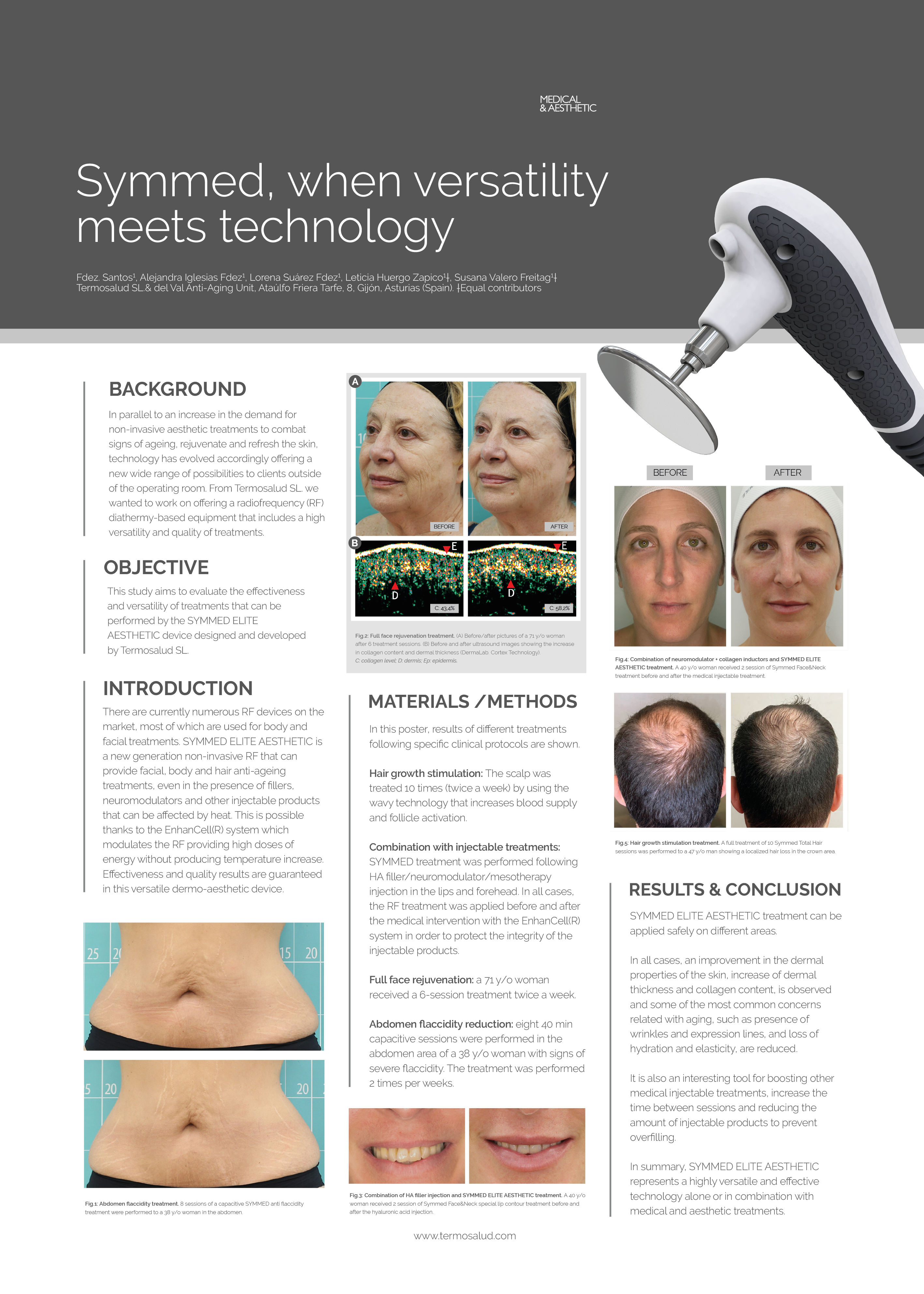
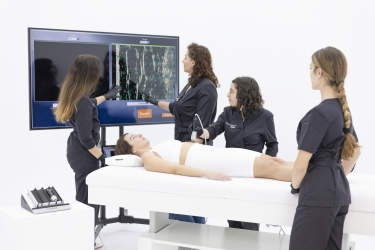
A space reserved for the management and development of studies where the different participant profiles are treated with our technologies and work protocols. The results obtained and their scientific analysis allow us to determine their efficacy, implement improvements and guarantee the absence of side effects or treatment risks.
Scientific research
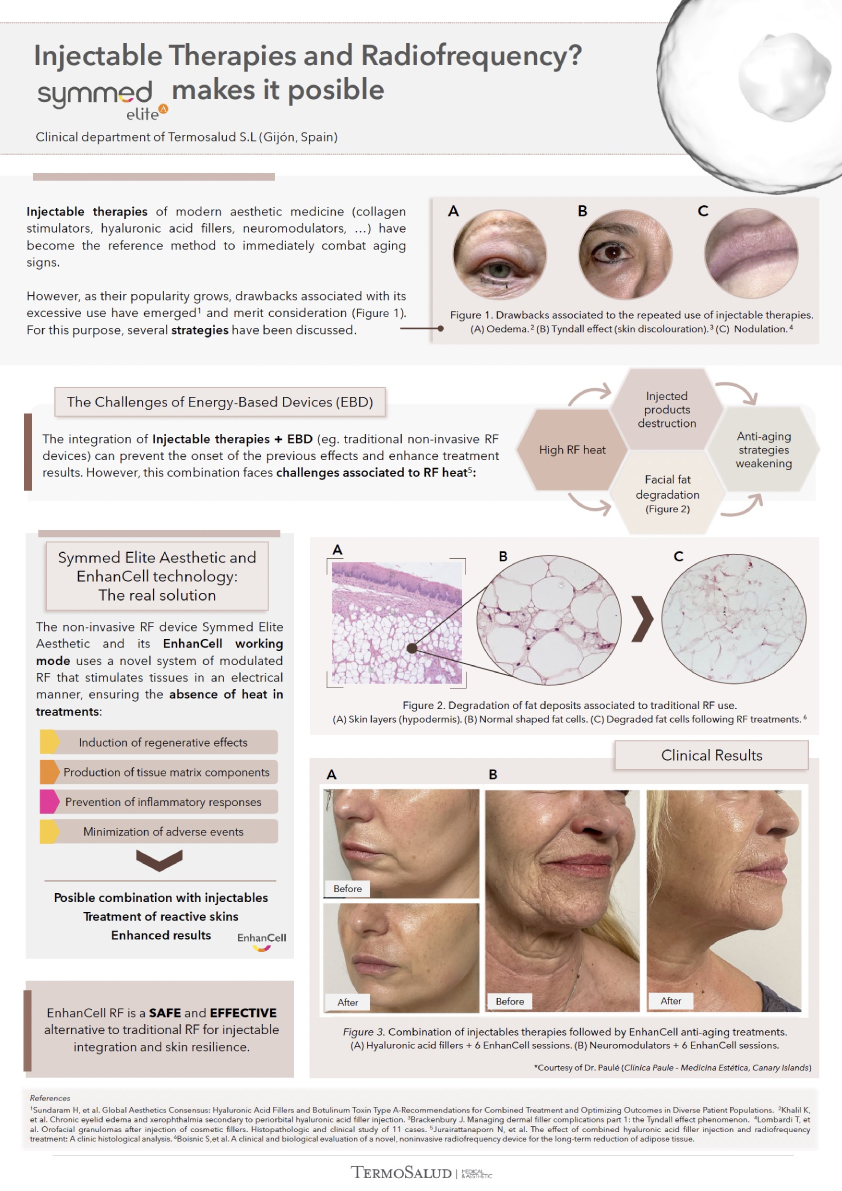
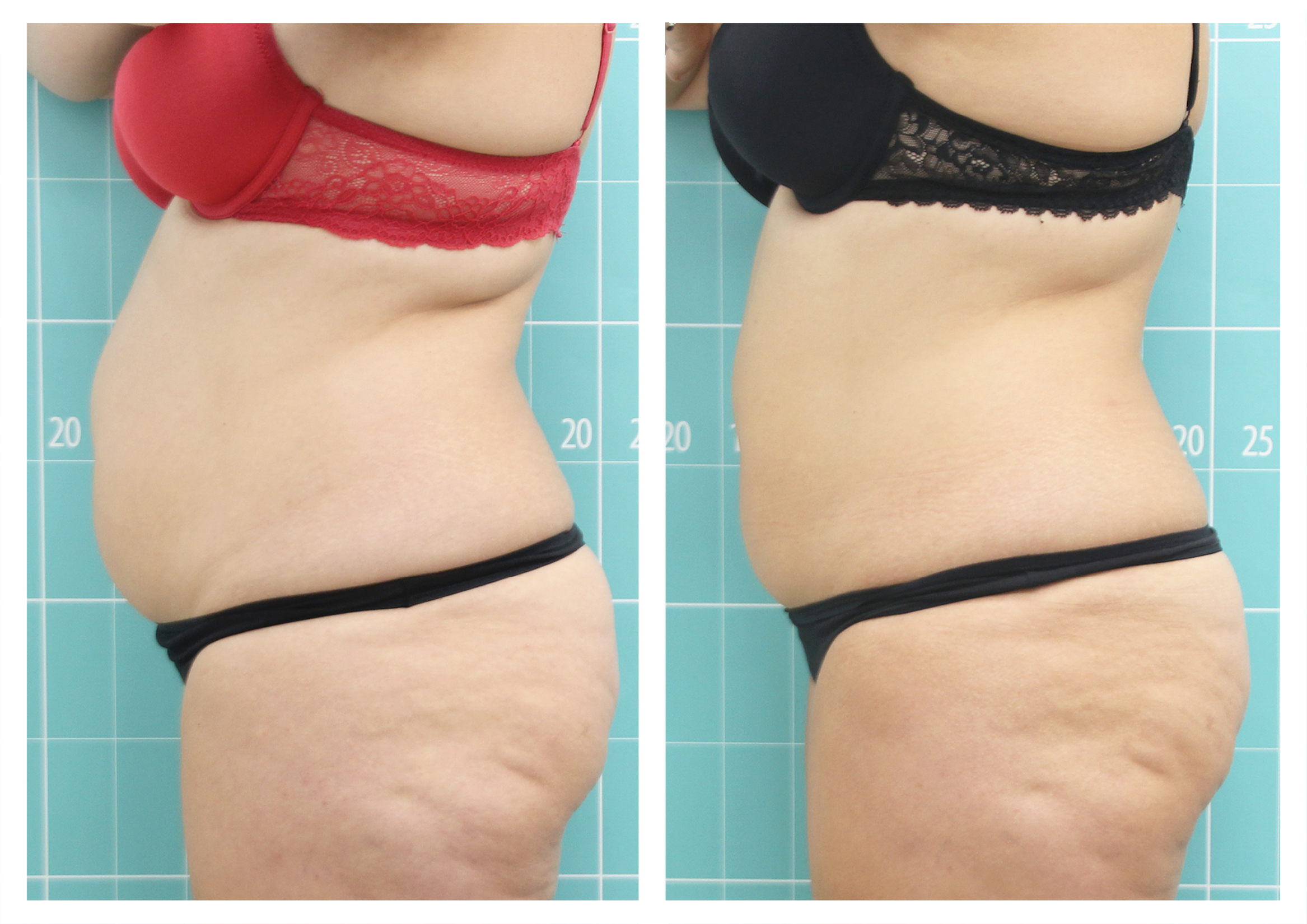
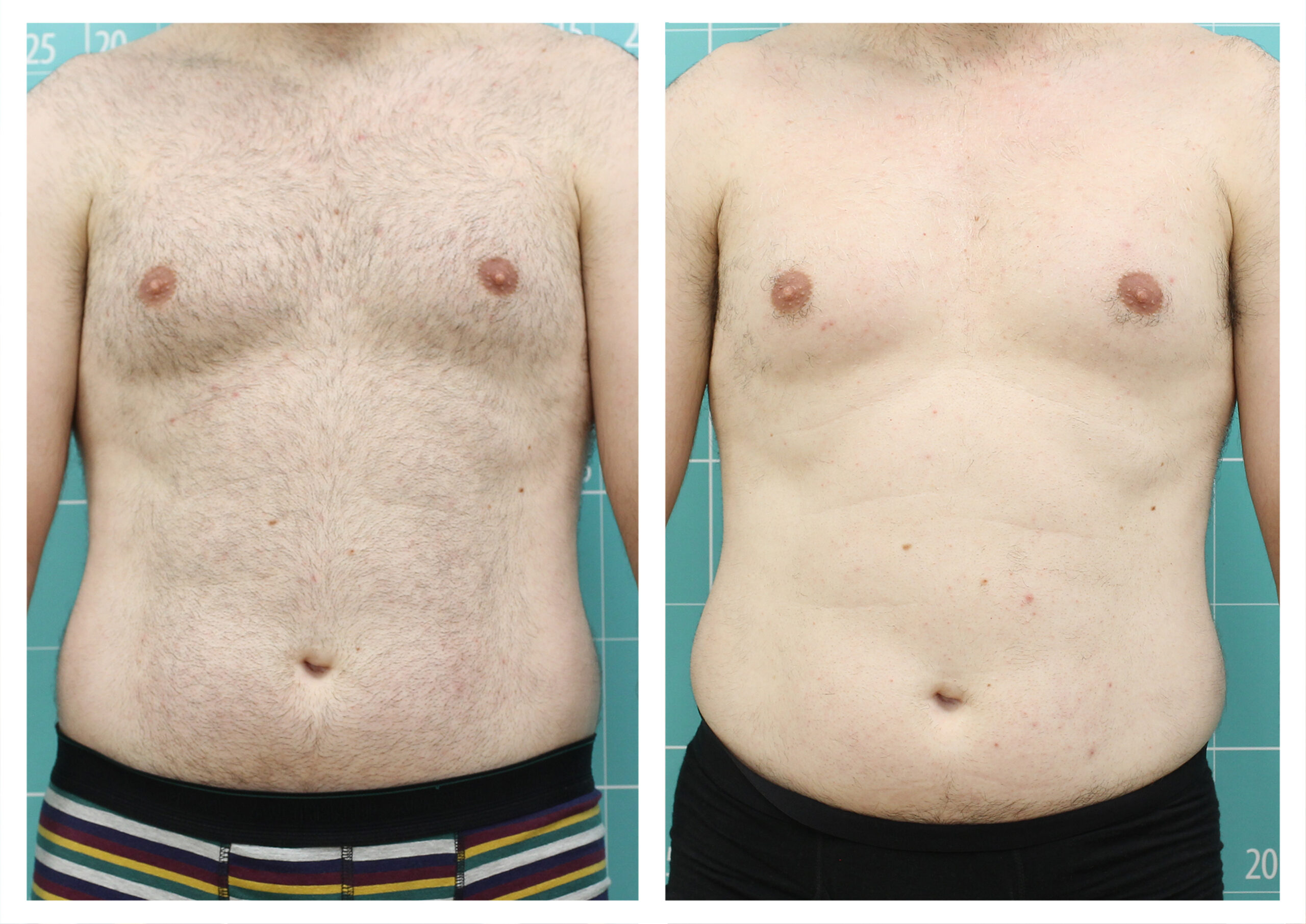
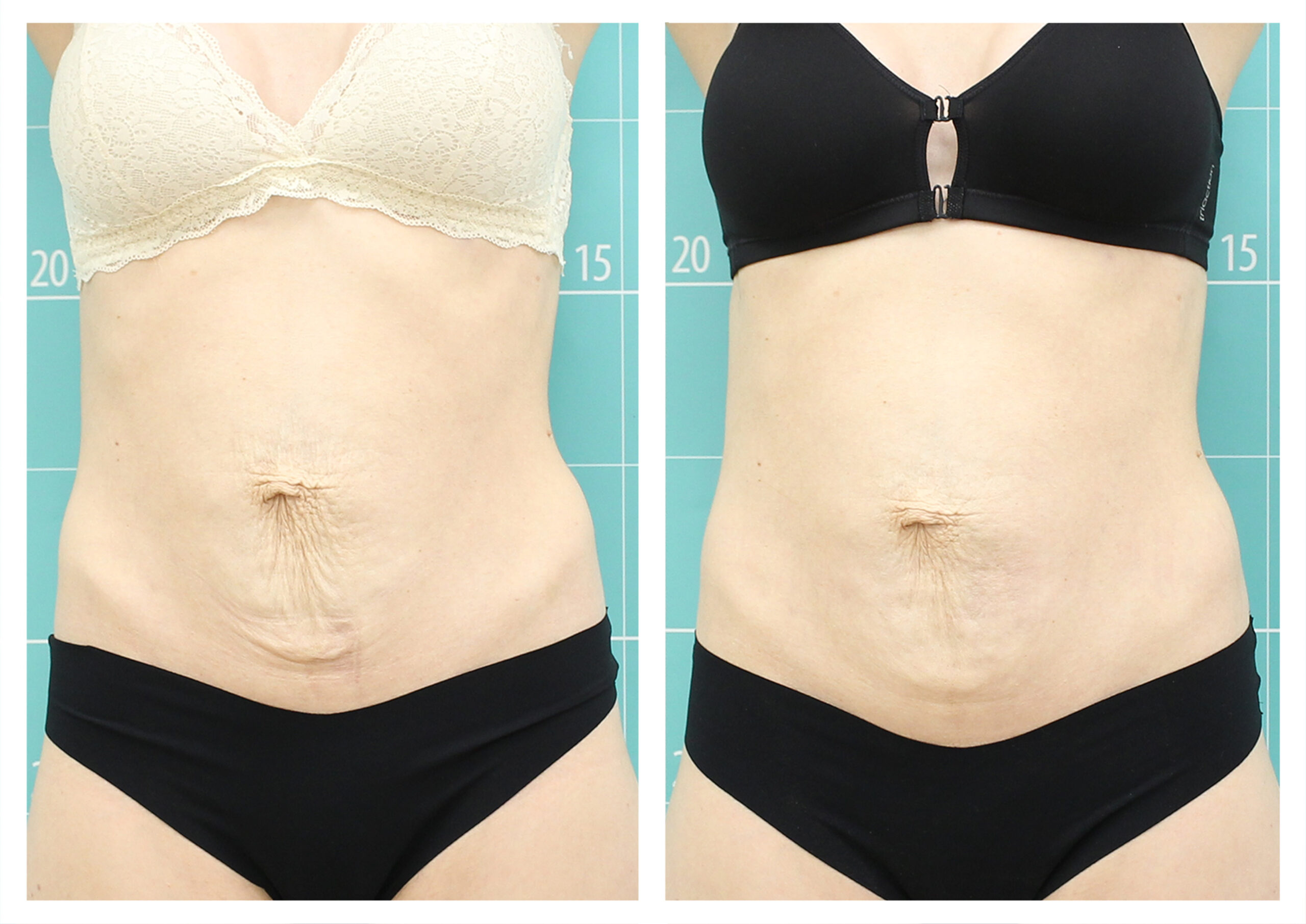
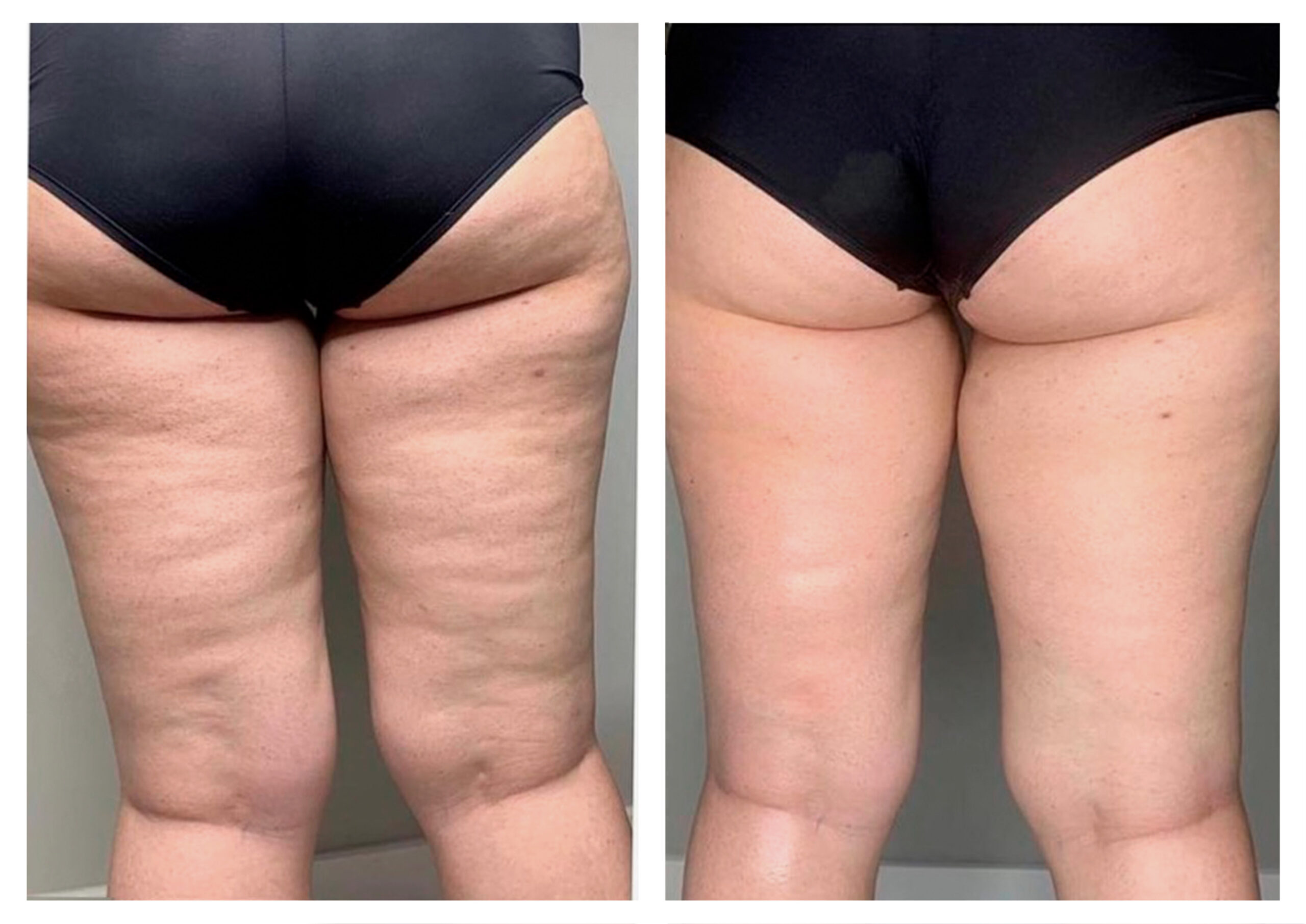
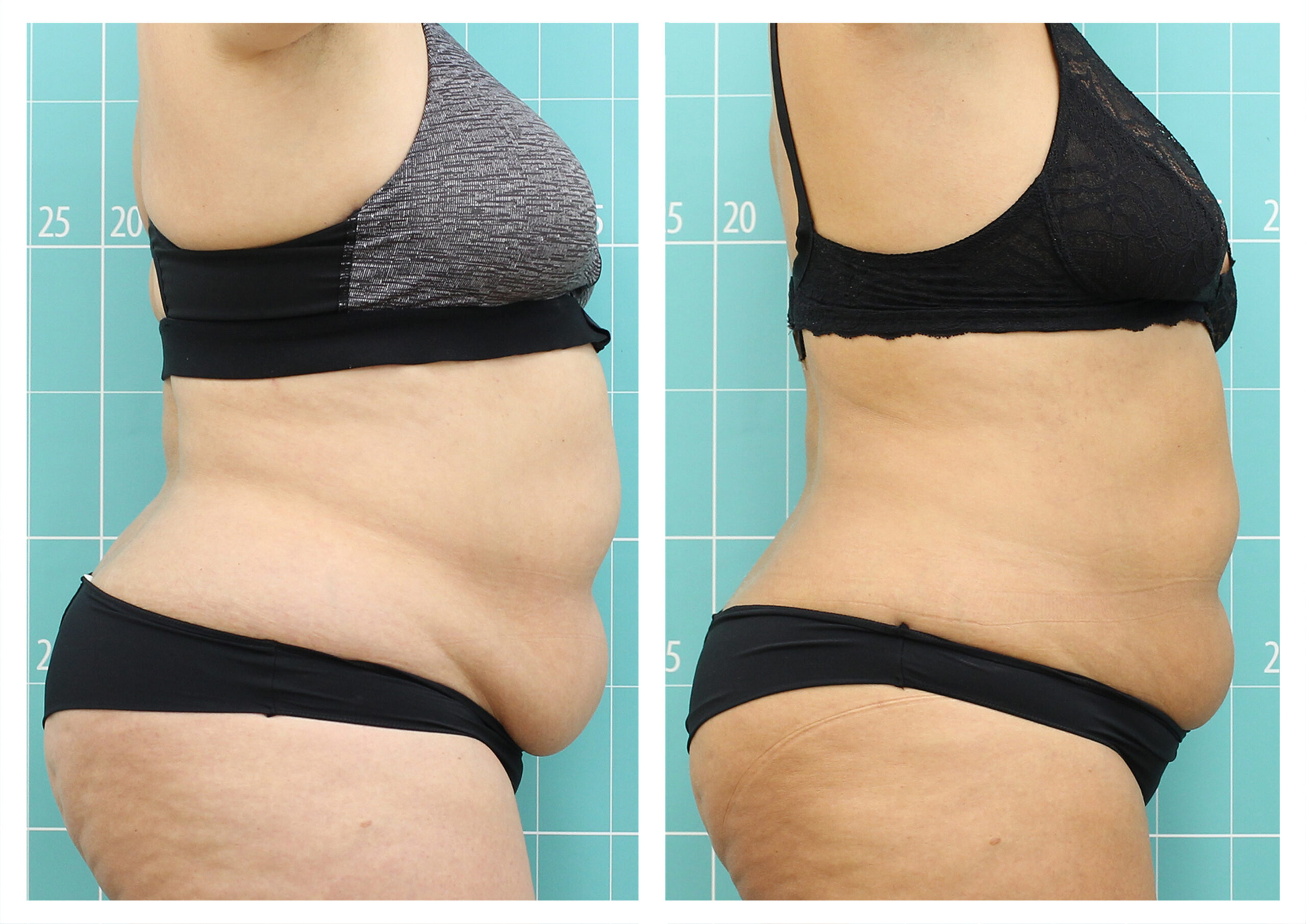
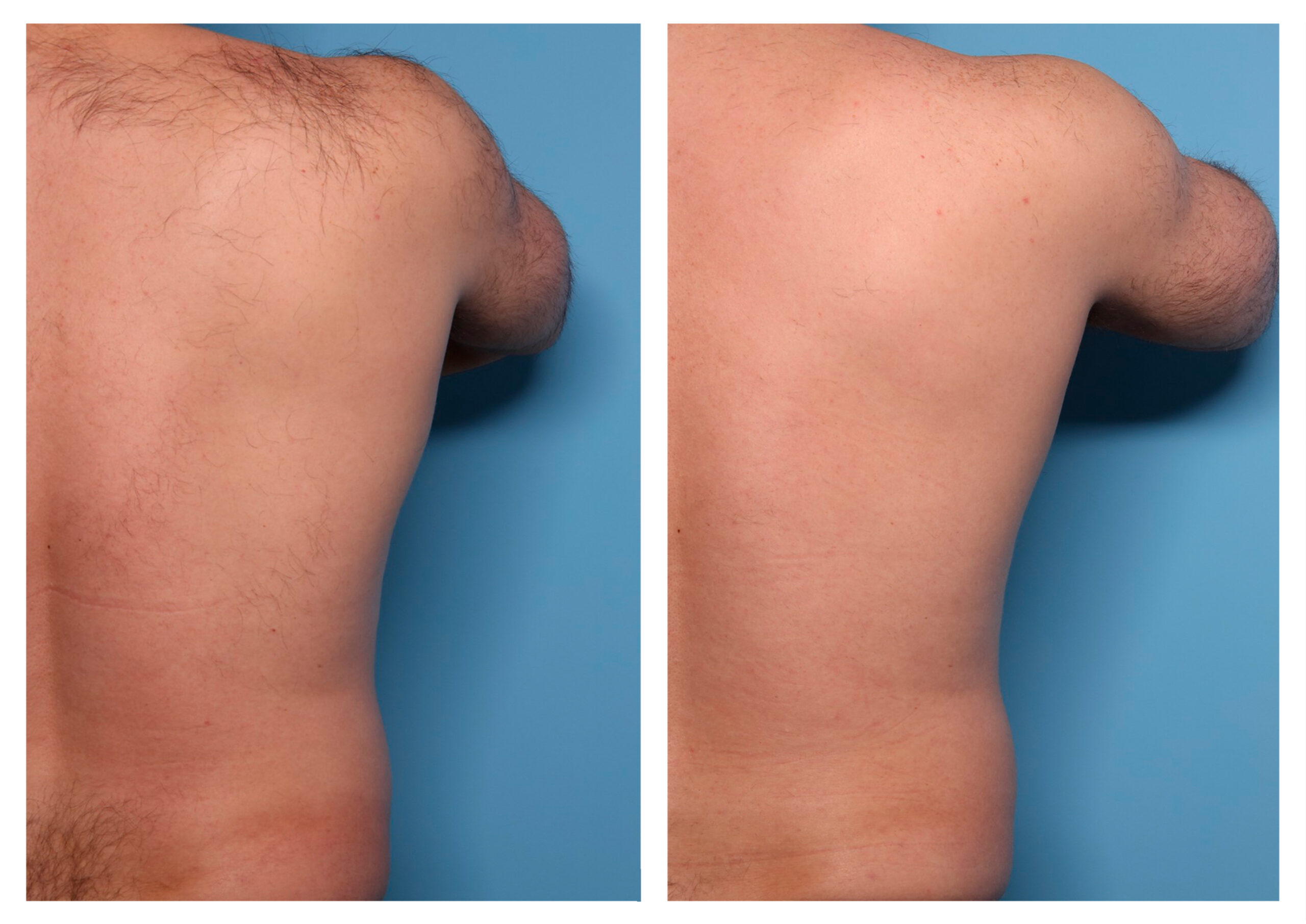
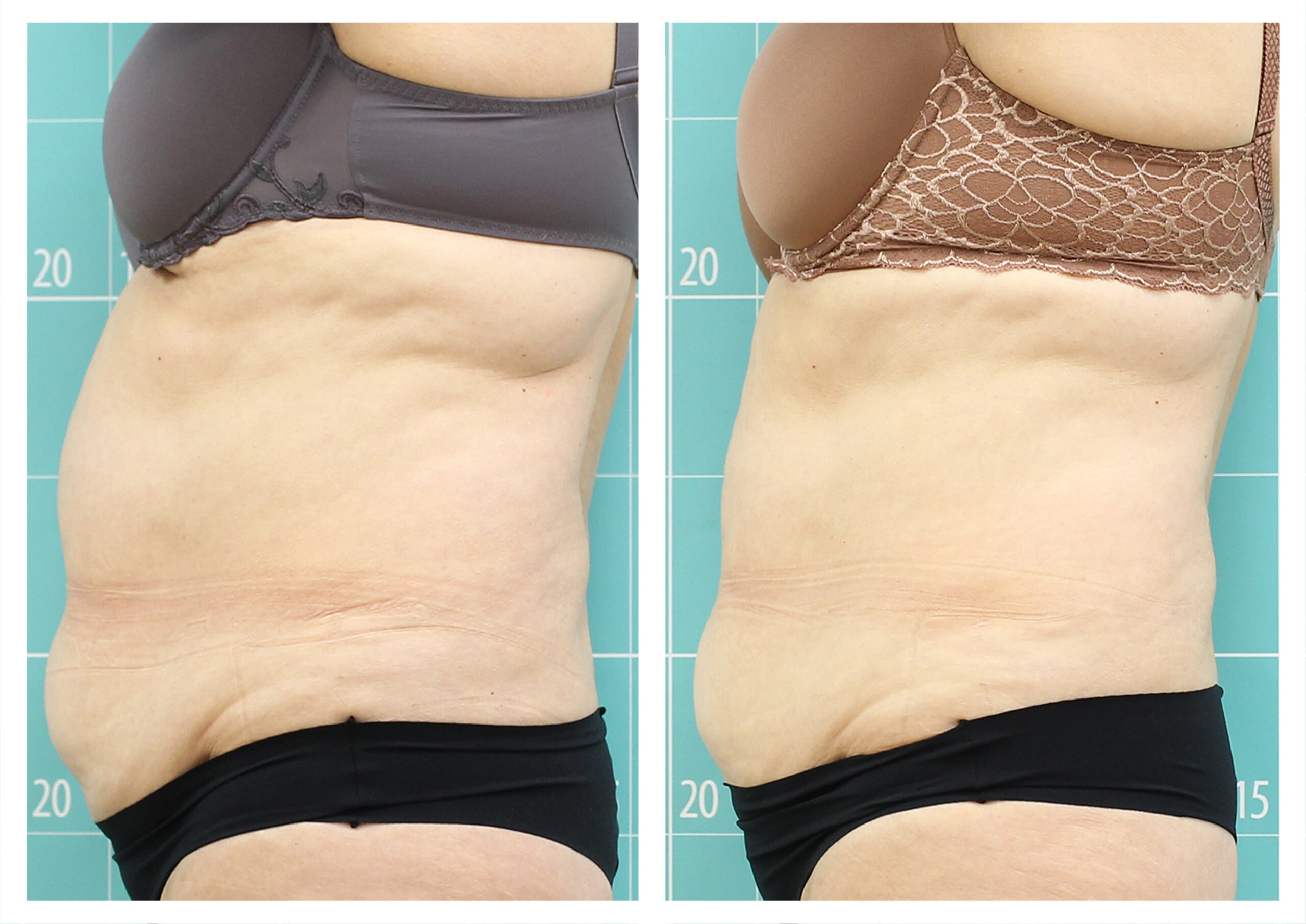
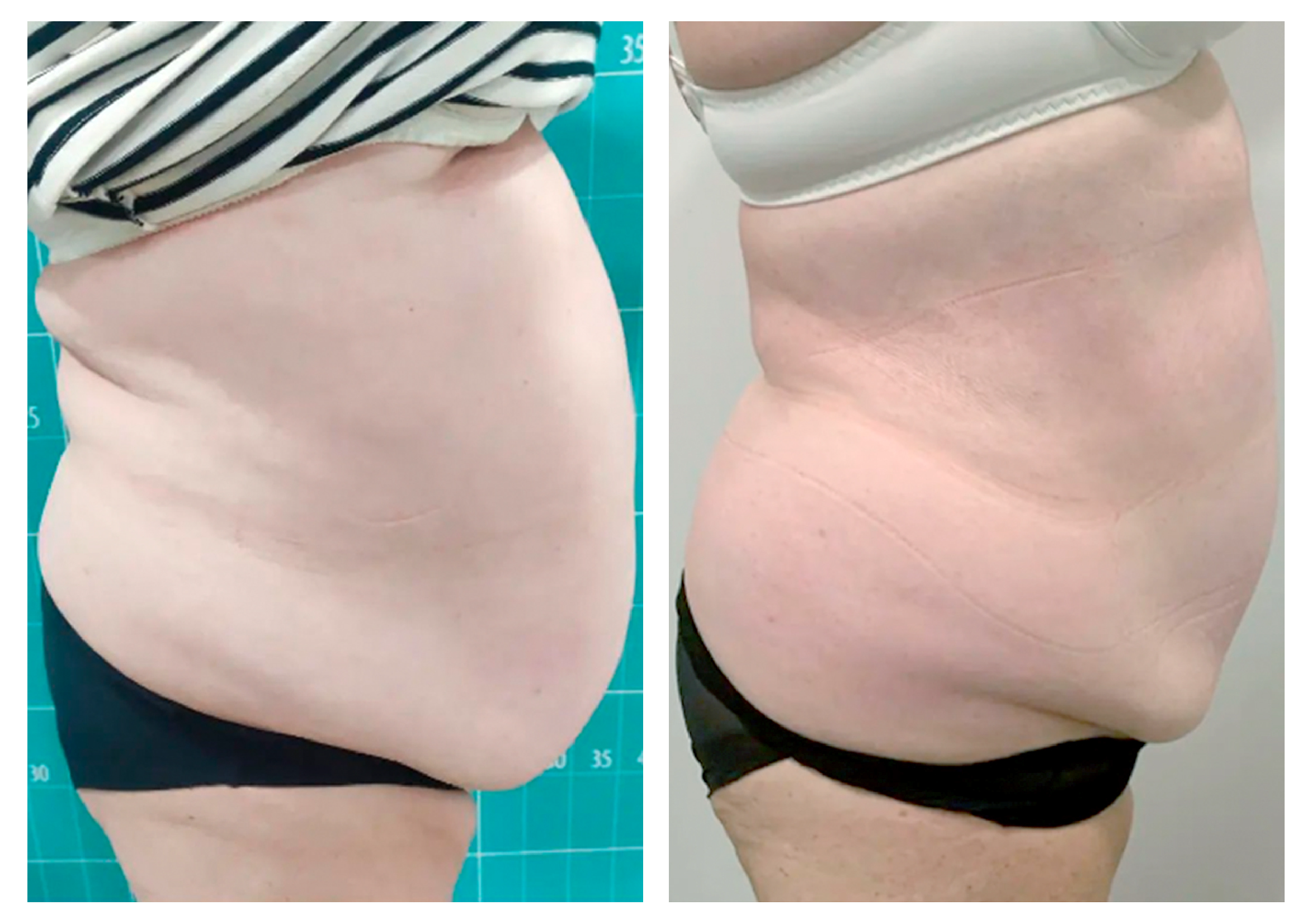
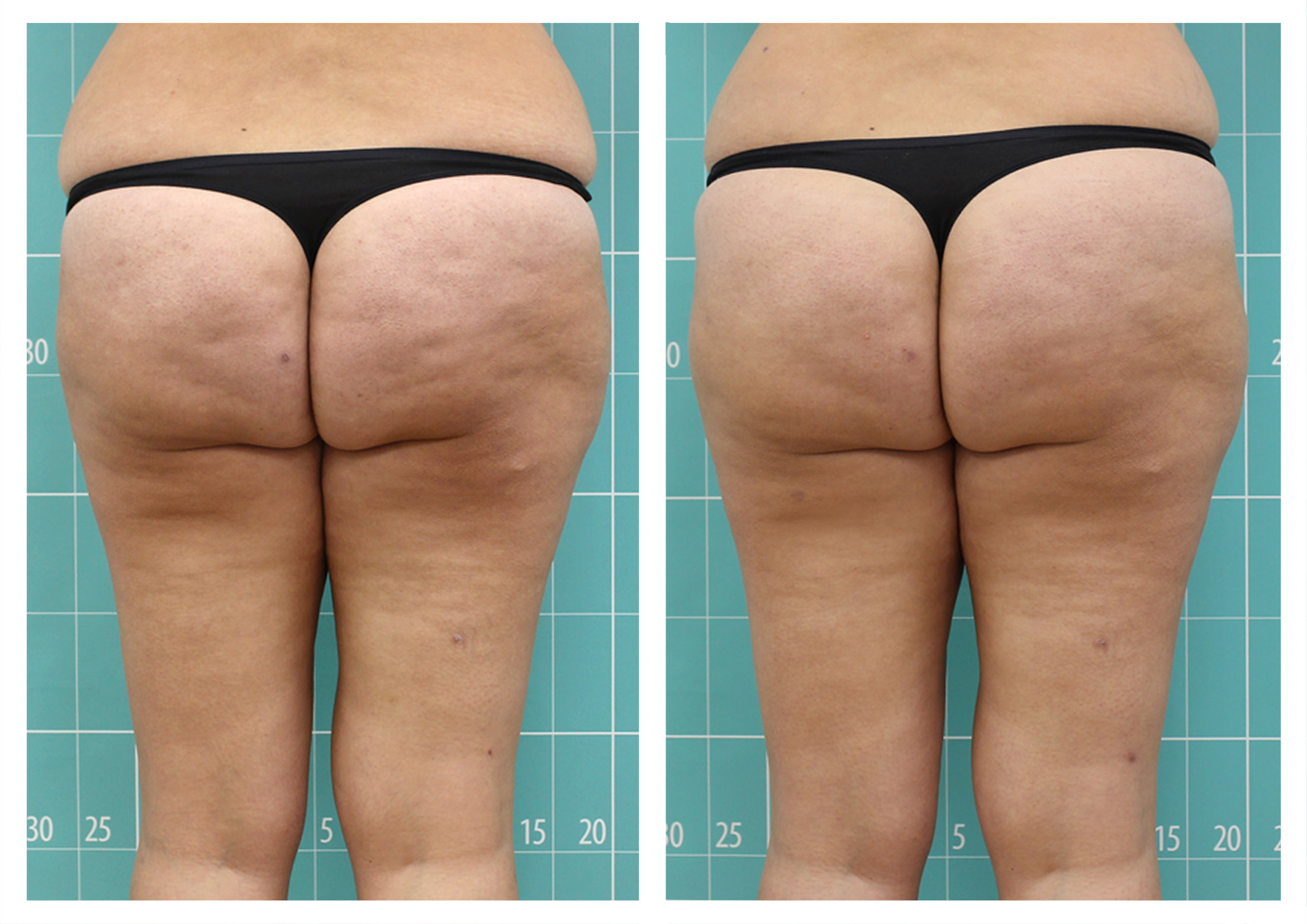
- Rotational radiofrequency-based technology leads to adipose tissue reduction and contouring effect in the thighs, abdomen, and flanks. [Published]
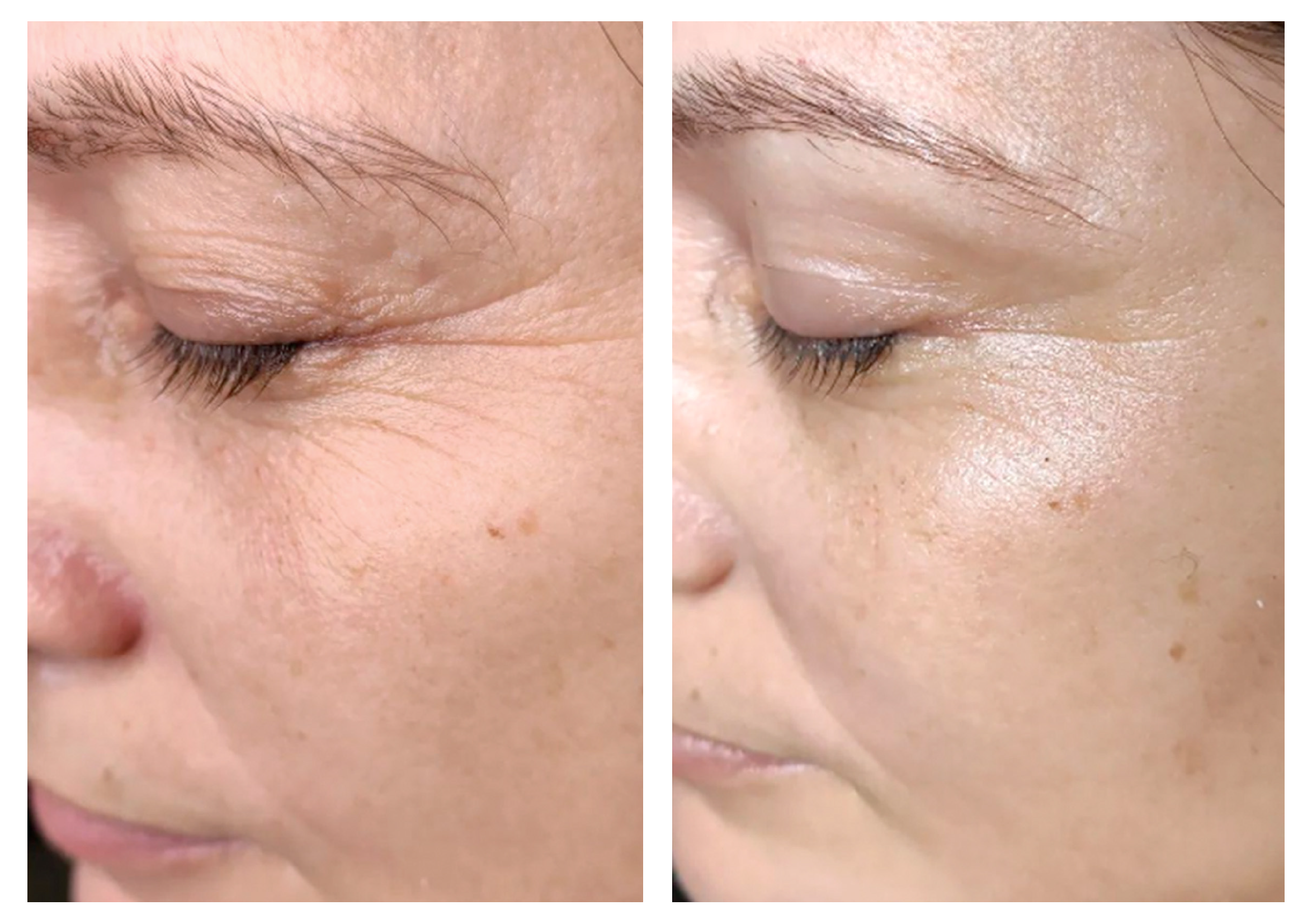
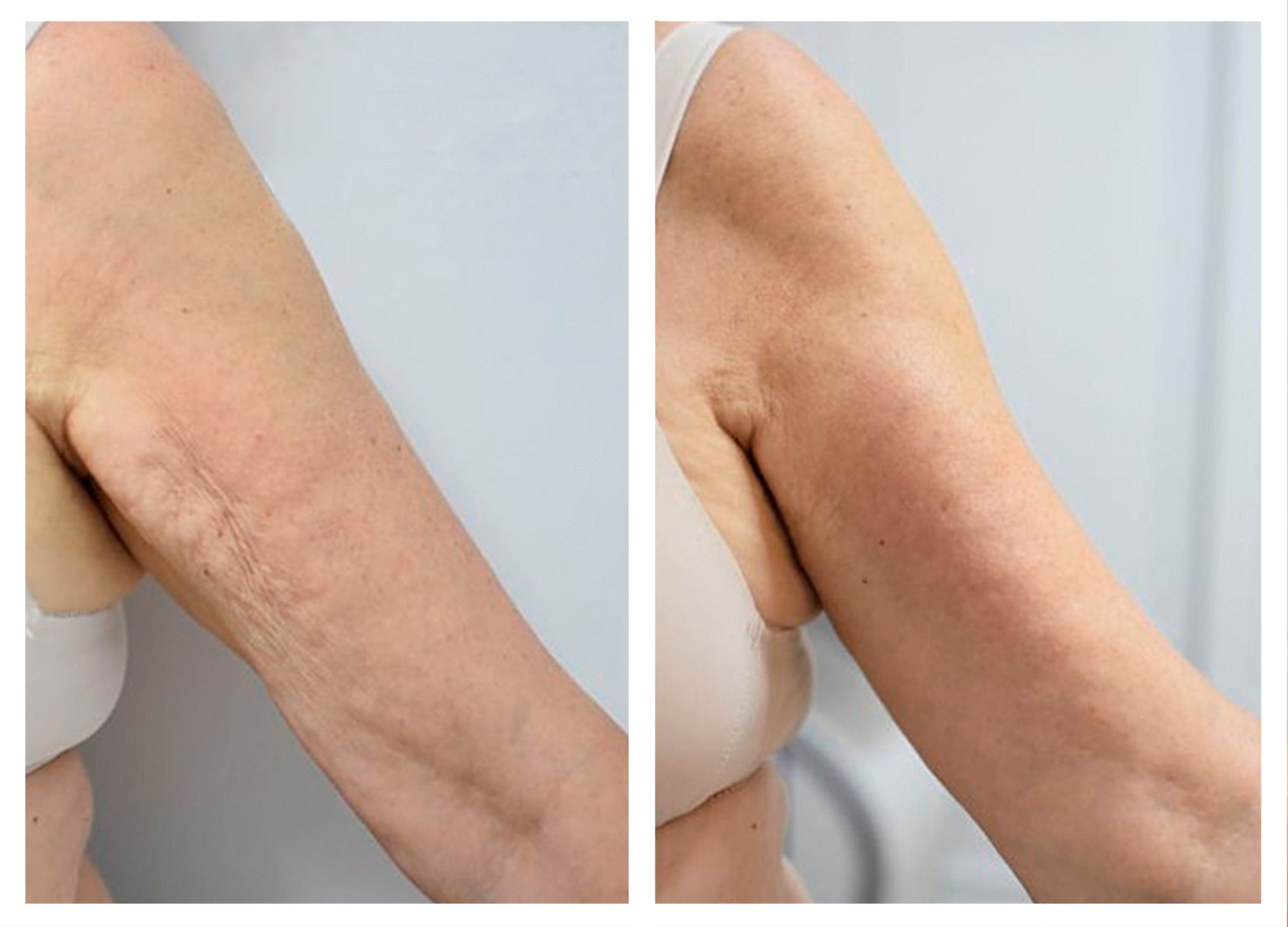
- Effectiveness of body remodeling and cellulite appearance improvement treatments in the thighs using Symmed radiofrequency device. [Published]
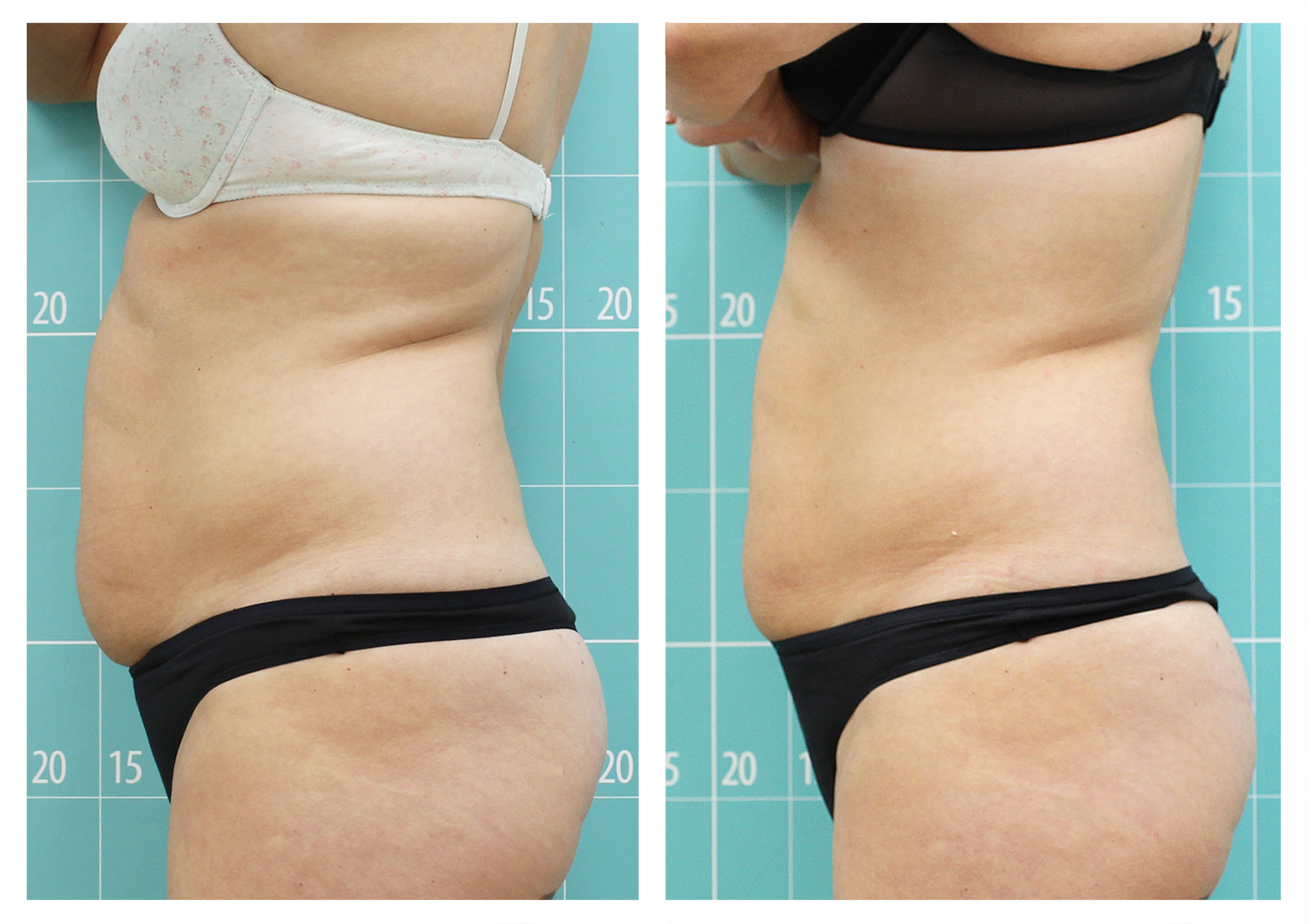
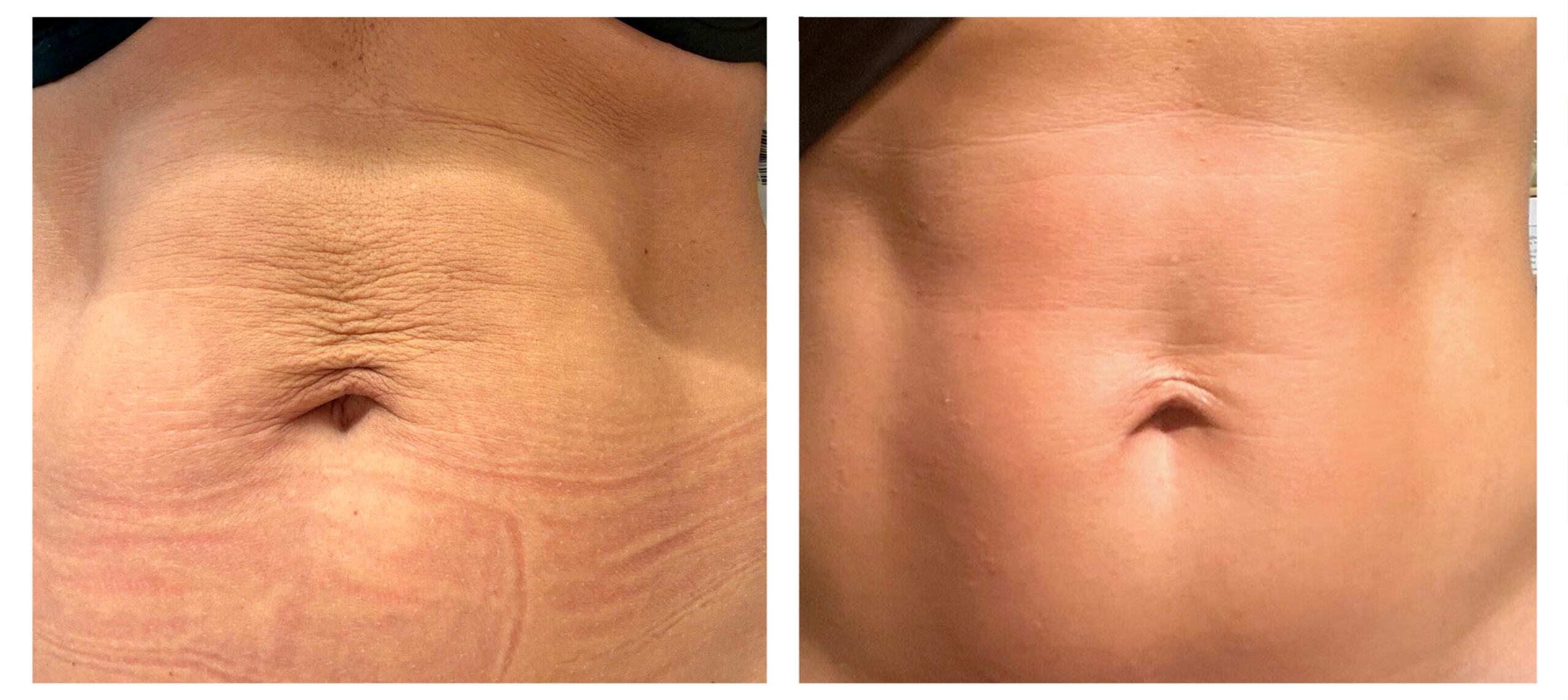
- Body shaping and skin appearance improvement in the abdomen and flanks by Symmed radiofrequency technology. [Under revision]
Use of biocompatible cosmetics and materials
Cytotoxicity, skin sensitisation and irritation tests according to the highest standards and current ISO 10993 regulations to ensure safe and effective skin contact.
Methodology
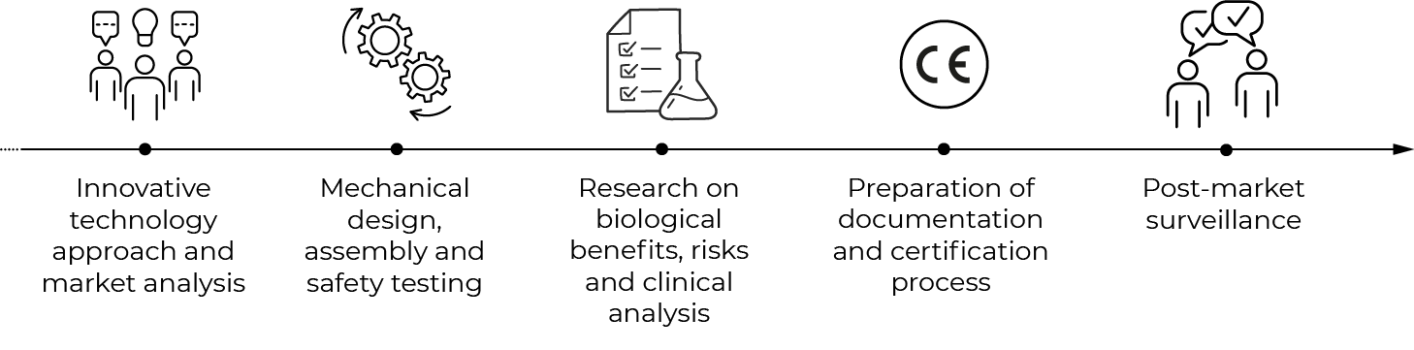
Each of our technologies is associated with a rigorous design and development process divided into several phases.
Clinical tools

Thermographic plates to determine the presence, biological characteristics and improvement of sclero-fibro-oedematous panniculopathy (cellulitis).


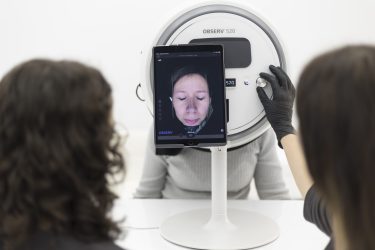
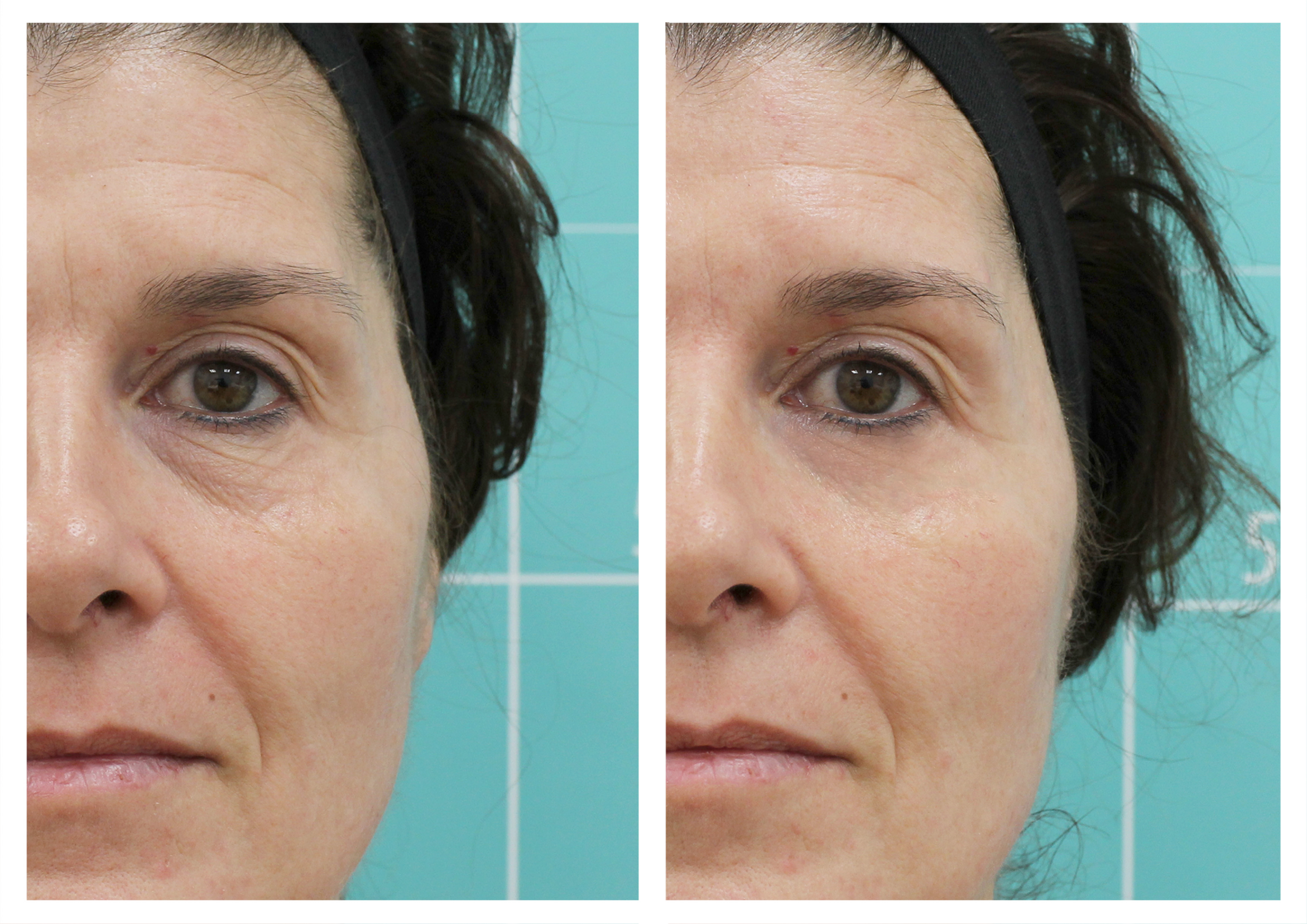
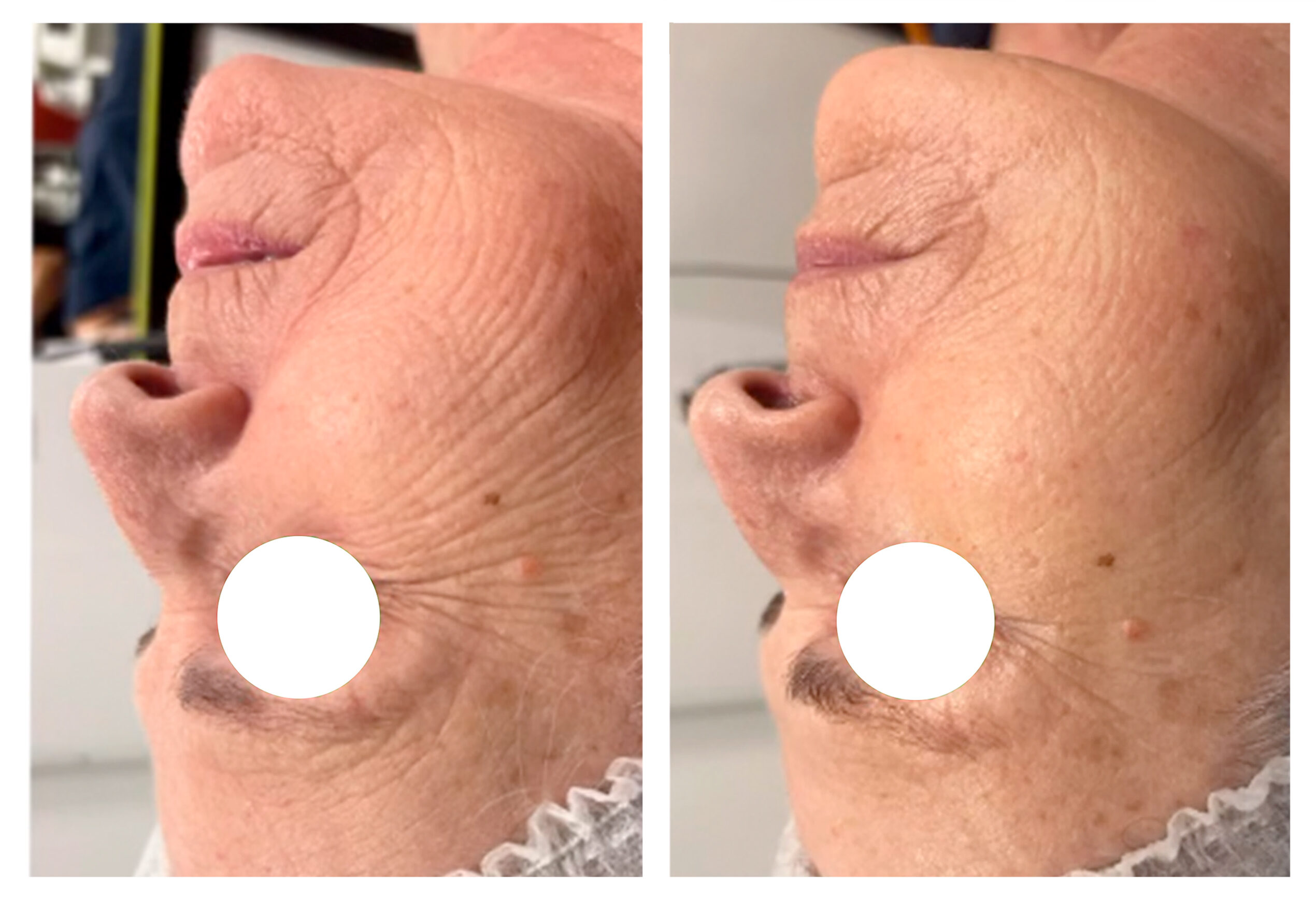
Assessment of skin disorders such as moisture loss, sun damage and redness.



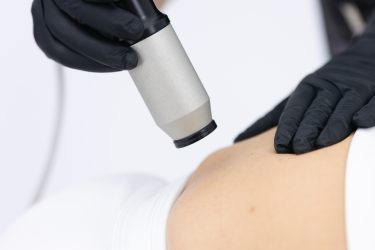
Facial ultrasound probe for a comprehensive analysis of the thickness of the fat layer, collagen content and elasticity level of the skin.
Other projects



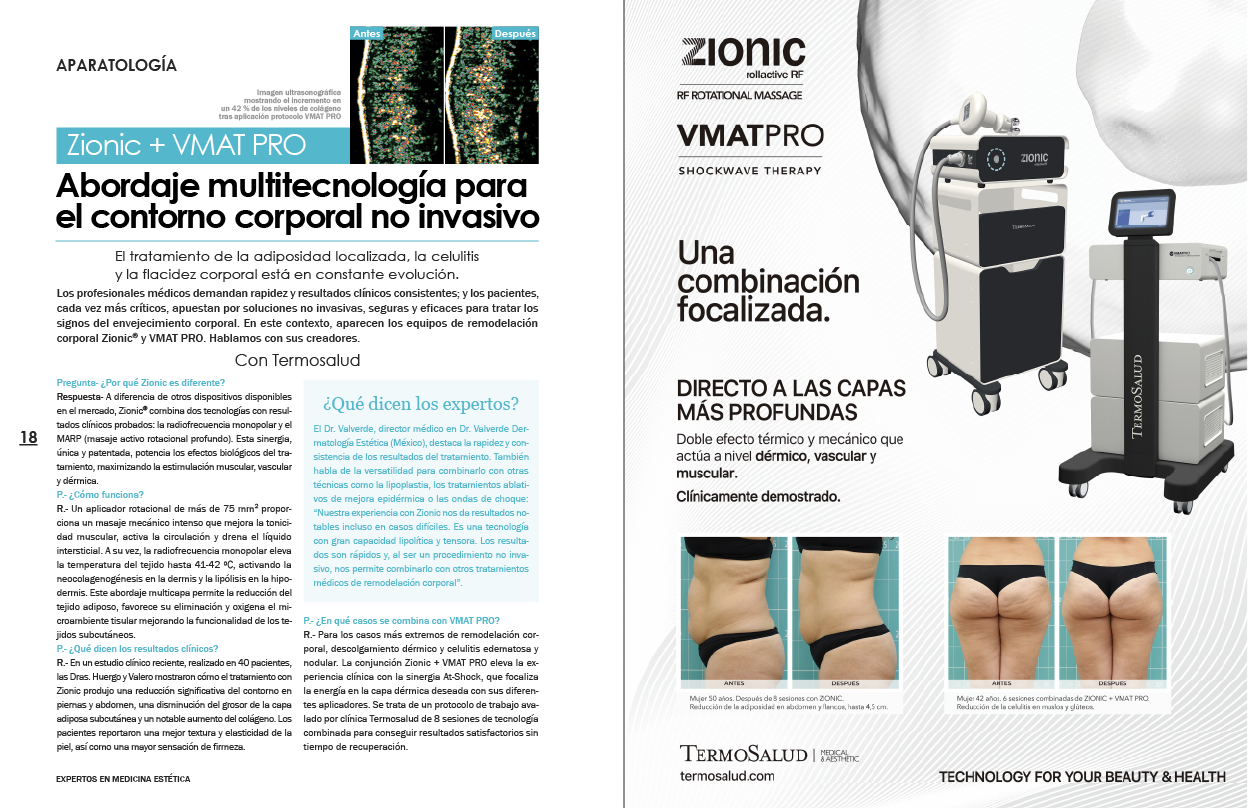
What do our doctors think?




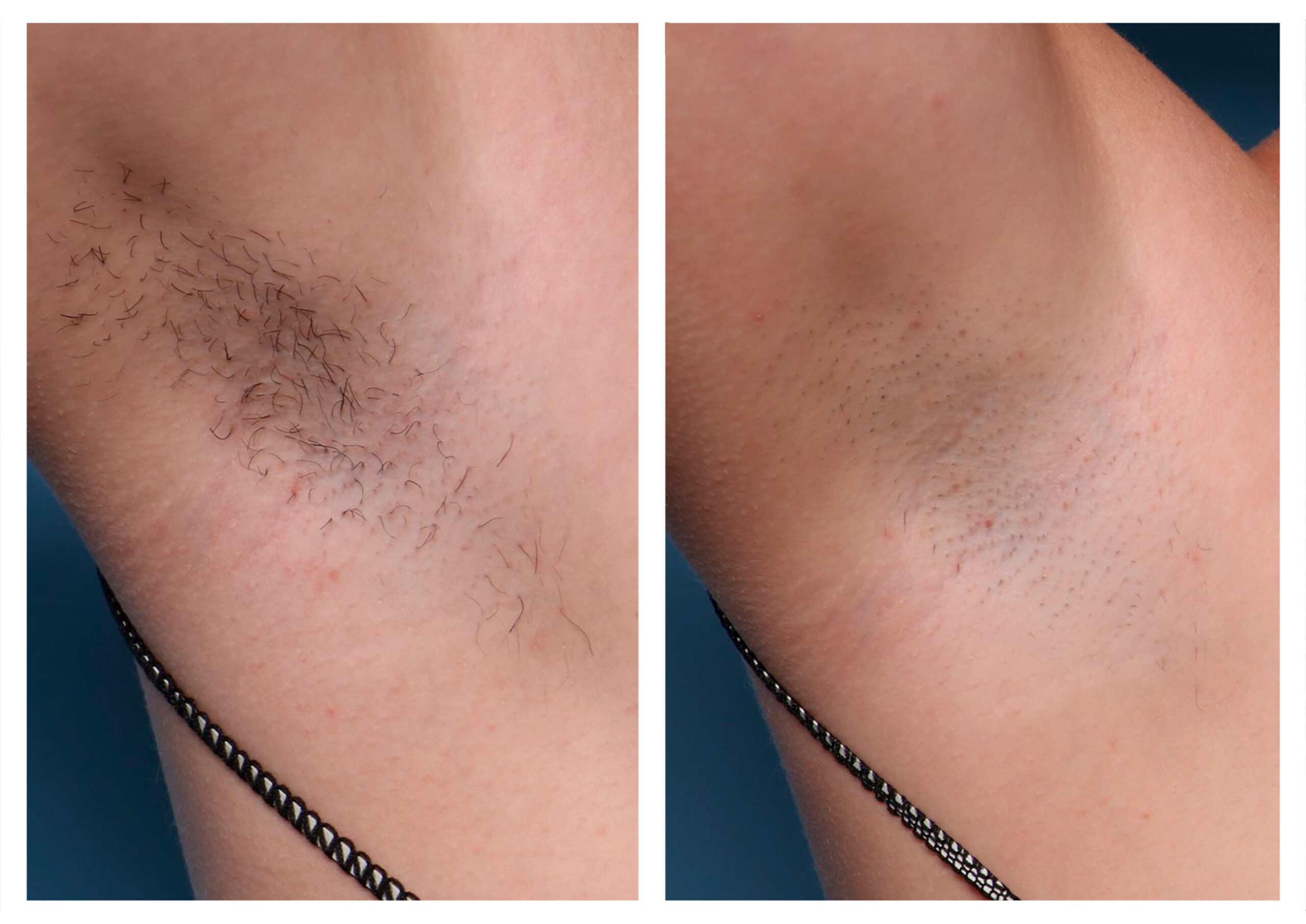
RESULTS

CLINICAL
COMMITMENT
Termosalud has a clinical department, staffed by doctors and experts in the sector who evaluate and guarantee the efficacy and safety of our medical and medical-aesthetic devices on a daily basis.
Our clinical commitment ranges from rigorous compliance with the ISO 13485 standard referring to the quality management system applicable to medical devices, to the development of scientific studies monitored by the Ethical Committee of Asturias and in compliance with the Declaration of Helsinki.
TERMOSALUD_Clinic
A space reserved for the management and development of studies where the different participant profiles are treated with our technologies and work protocols. The results obtained and their scientific analysis allow us to determine their efficacy, implement improvements and guarantee the absence of side effects or treatment risks.
- Rotational radiofrequency-based technology leads to adipose tissue reduction and contouring effect in the thighs, abdomen, and flanks. [Published]
- Symmed radiofrequency contributes to adipose tissue reduction and collagen increase in thighs. [In progress]
- Body shaping and skin appearance improvement in the abdomen and flanks by Symmed radiofrequency technology. [Under revision]
Cytotoxicity, skin sensitisation and irritation tests according to the highest standards and current ISO 10993 regulations to ensure safe and effective skin contact.
Methodology
Each of our technologies is associated with a rigorous design and development process divided into several phases.
Clinical tools

Thermographic plates to determine the presence, biological characteristics and improvement of sclero-fibro-oedematous panniculopathy (cellulitis).



Assessment of skin disorders such as moisture loss, sun damage and redness.



Facial ultrasound probe for a comprehensive analysis of the thickness of the fat layer, collagen content and elasticity level of the skin.


Other projects




What do our doctors think?
Dra. Lupian
"ZIONIC has become the best body treatment. The results on grade III cellulite and localised fat are incredible. Patients are delighted. ZIONIC has a high therapeutic value as it is a comfortable treatment and can treat the patient in a comprehensive way".

Dr. Miguel Paule
"With SYMMED ELITE AESTHETIC, I have improved the results obtained with hyaluronic acid fillers and collagen-inducing treatments. Two weeks after any injectable treatment, we recommend six sessions of SYMMED ELITE AESTHETIC on the neck, décolleté and face, once or twice a week. The EnhanCell system, which allows for athermic conditions, made me choose SYMMED ELITE AESTHETIC after thread lift treatments. The PowerCell system works in pulsed hyperthermia, ensuring absolute control over the treatment".

Dr. Balmori
"The ZIONIC medical team has transformed the Balmori Aesthetic Centre by elevating the quality of treatments and patient satisfaction. This innovative technology has proven to be a powerful duo in the quest for healthier, more radiant skin, in line with the clinic's ongoing commitment to excellence and innovation in aesthetic medicine".

Dra. Iratxe Díaz
"I have known Termosalud for almost 20 years and they have never let me down. In my practice I have their VMAT PRO and ZIONIC equipment , with which we safely and effectively treat cellulite, flaccidity, fat accumulation and signs of skin ageing. I am grateful for the technology, but also for the friendly treatment and the efficiency of Termosalud, which is always there when we need it".

Dr. Valverde
"Our experience with ZIONIC gives us remarkable results even in difficult cases. It is a technology with a great lipolytic and firming capacity. The results are fast and non-invasive, which allows us to combine it with other medical treatments for body remodelling".

RESULTS


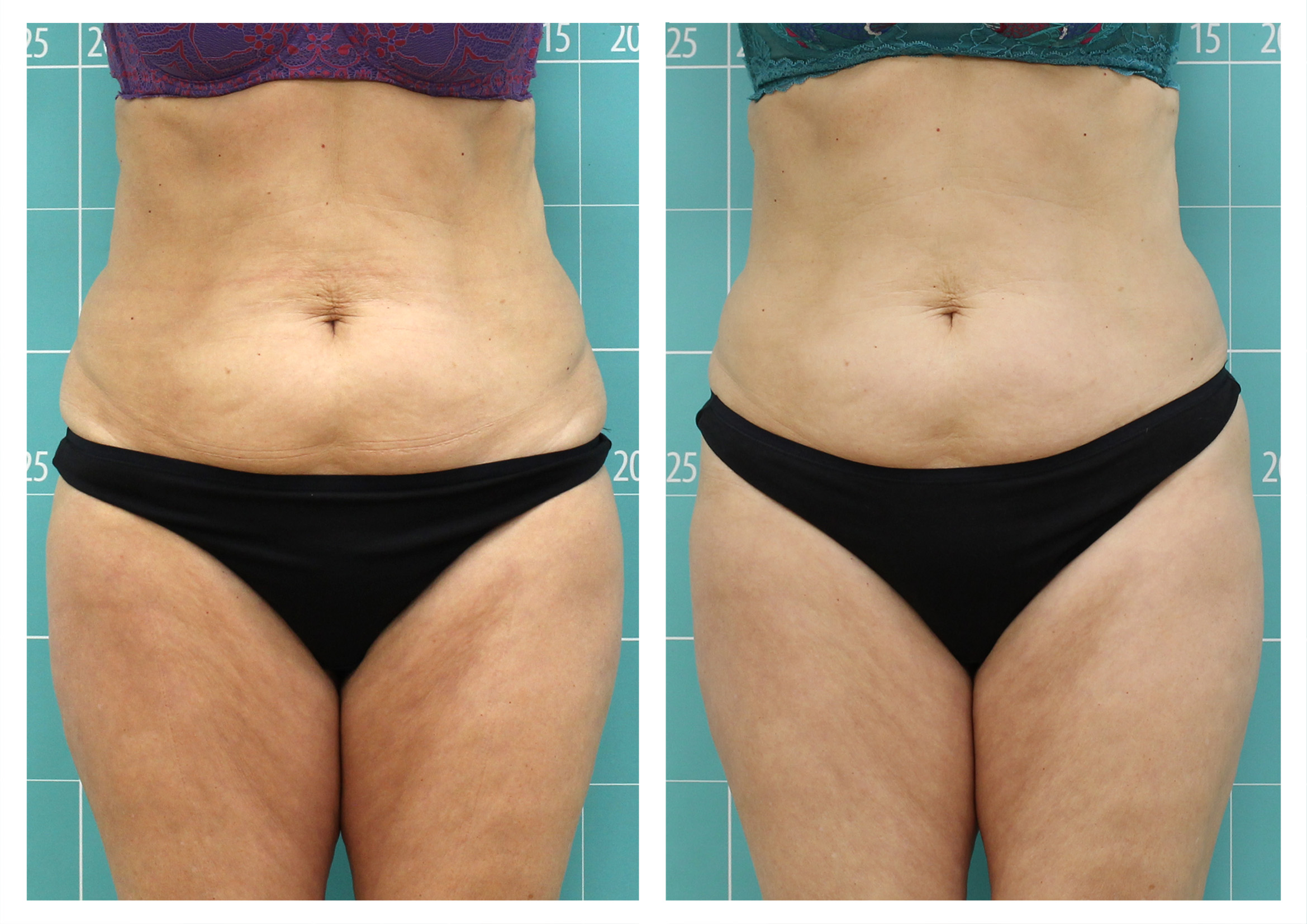
Clínica Termosalud



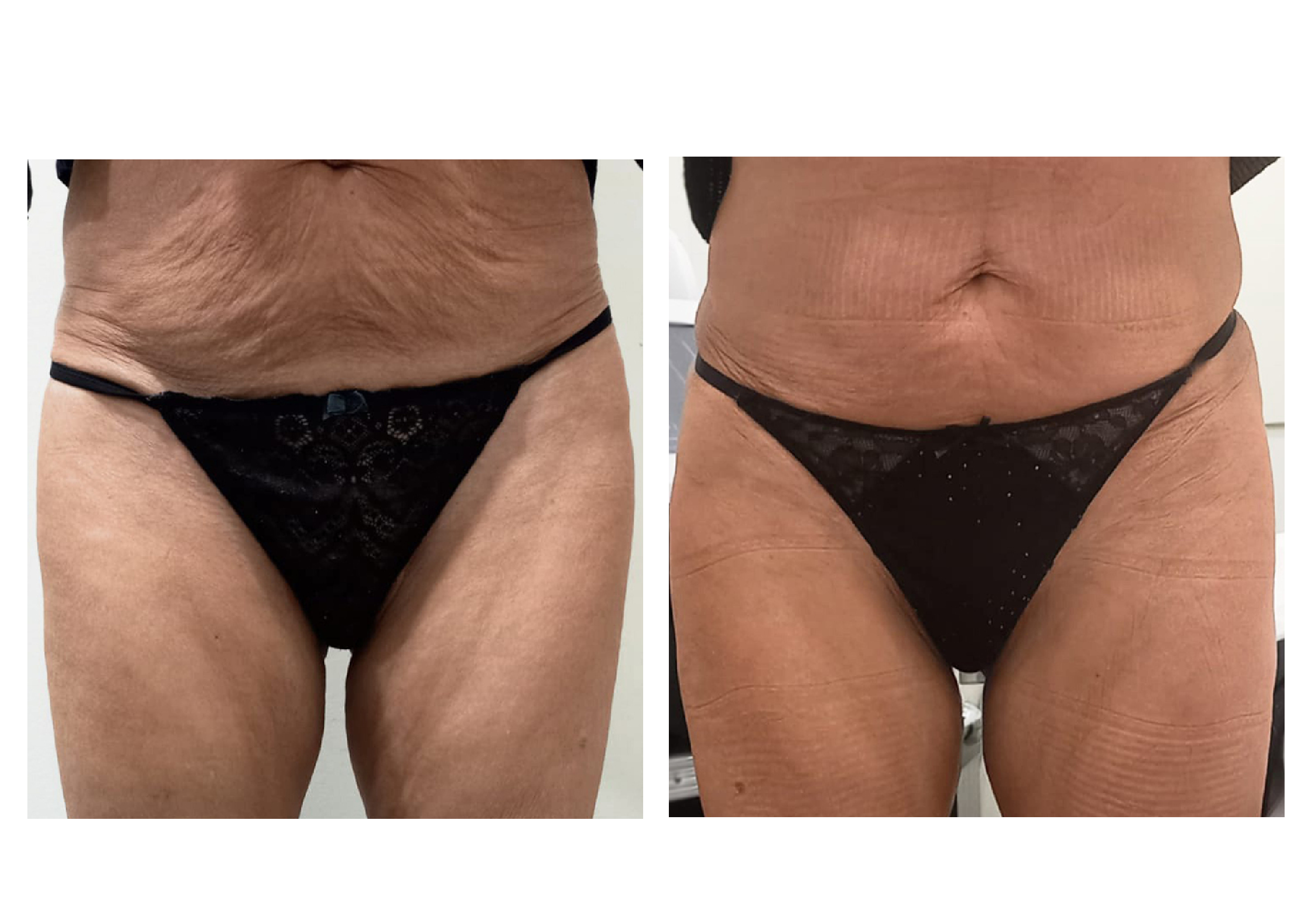
Nova Estètica Lleida




Candela Medical
RESULTS















NEWS
Termosalud workshop specialised in SPMEC Oporto
During the IV National Congress of Aesthetic Medicine in Porto,...
Leer más